Axel H. E. Müller, Marvin Steube, Tobias Johann, Dominik Fuchs, Thi Dinh,
Christoph Hahn, Moritz Meier-Merziger, Shivani Wadkonkar, Holger Frey.
Department of Chemistry, Johannes Gutenberg University Mainz, 55099 Mainz, Germany
The statistical anionic copolymerization of styrene (S) with 1,3-dienes with lithium counterion in apolar solvents is commonly used to synthesize “tapered” block copolymers, enabling the control of the phase behavior by adjusting the order-disorder transition temperature. Lewis-base modifiers (ethers, amines) have been used to “randomize” the copolymerization, also affecting the regio- and stereostructure of the polydiene comonomer segments.
Recently, biobased terpene derivates like β-myrcene or β-farnesene have come into the focus of interest. These building units can be obtained directly from natural feedstocks, by enzymatic conversion of biomass or indirectly by chemical transformation processes.
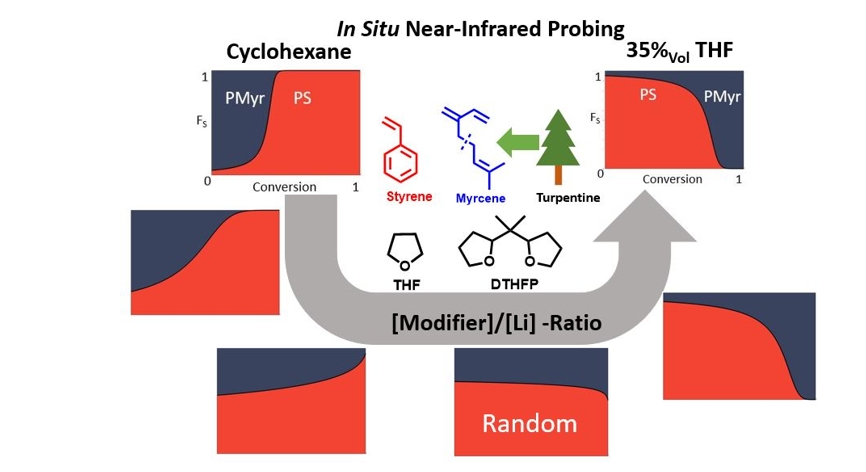
The effect of mono- and multidentate ethers on the copolymerization kinetics of styrene with isoprene, myrcene and farnesene, respectively, and the gradient of the resulting copolymers were systematically investigated by increasing the [modifier]/[Li] ratio). In situ near-infrared (NIR) spectroscopy was employed as a versatile and fast method to track the highly accelerated consumption of the individual monomers. Reactivity ratios were determined as a function of the [THF]/[Li] ratio. Corresponding changes in the copolymer composition profile up to a complete inversion are evident in thermal properties and morphologies. Although all copolymers possess the same comonomer composition (57%vol PS-units) SAXS and TEM give evidence of a wide variation in bulk morphologies depending on the gradient profile.
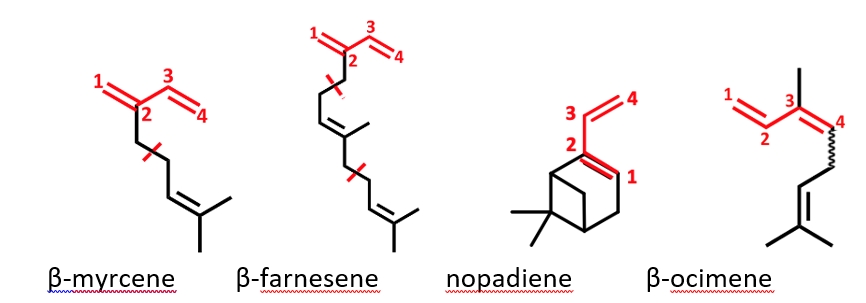
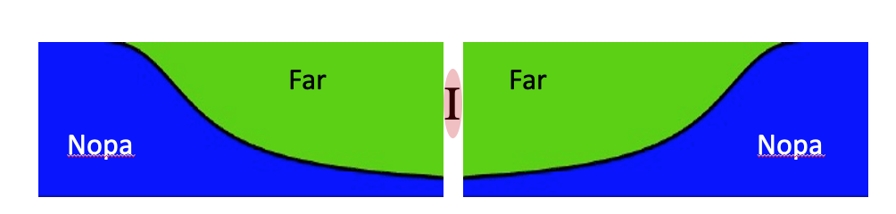
Nopadiene (Nopa) is a new terpene-based diene monomer. PNopa obtained by anionic polymerization in cyclohexane or MTBE has a surprisingly high Tg of 160 °C, potentially replacing sttyrene. Thus, ABA block copolymers with other dienes are thermoplastic elastomers (TPEs). Based on favorable reactivity ratios the statistical copolymerization of Nopa and farnesene using a difunctional initiator in MTBE renders TPEs within just one single step.
We also investigated the terpene-based diene monomer β-ocimene (Oc). It is a 3,4-substitued 1,3-diene, which comes as a mixture of cis- and trans isomers. It had not yet been explored in anionic polymerization. Real-time 1H NMR kinetics indicated a remarkably divergent reactivity of the isomers (kp,trans > kp,cis). This unveiled that the ‘homopolymerization’ of Oc is in fact a copolymerization of the cis and trans isomers. In contrast, in copolymerization of the isomers with styrene the reactivities are inversed (rtrans < rcis).
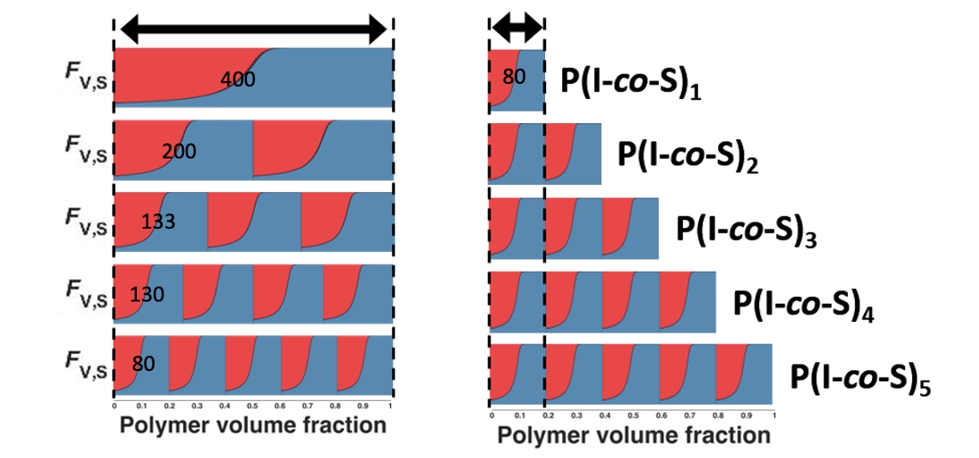
Using the knowledge obtained in the kinetic experiments we synthesized multiblock copolymers with up to ten blocks by sequential additions of the comonomer mixtures. The mechanical properties (viscoeleastic response) can be finely tuned by the judicious selection of molecular weight and number of blocks.
Universität Mainz, Germany