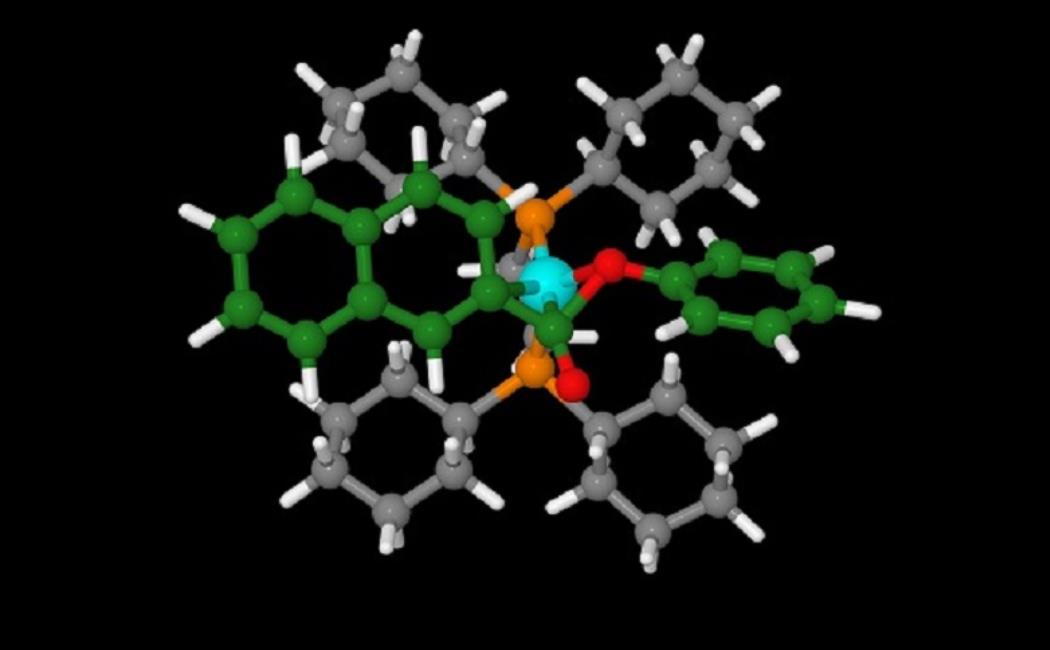
A bidentate ligand (orange and grey) on the nickel atom (blue) promotes the elimination of carbonyl in the ester (green and red) in this transition state.
A metal catalyst that gives distinct carbon-based molecular skeletons upon ligand change may unlock cost-effective, green synthetic routes.
A nickel catalyst is set to expand the chemist’s toolkit, leading to novel key intermediates in the synthesis of natural products and pharmaceuticals.
Researchers from KAUST have developed a synthetic approach that forms a carbon–carbon bond between a typically inert precursor and a boron-containing hydrocarbon, or alkyl borane, using a nickel catalyst.
Depending on the type of the ligands decorating the catalyst, this cross-coupling reaction produced alkyl-functionalized aromatic compounds. These bear chains comprising carbon and hydrogen atoms, or ketones, in which a double-bonded carbon–oxygen unit, known as the carbonyl group, is positioned between aromatic and alkyl parts.
Generally, cross-coupling reactions rely on palladium catalysts to generate carbon-based molecular skeletons. Most methods have aromatic compounds containing a halide, such as iodide or bromide, that are paired with aromatic boranes to build asymmetric molecules. However, these halide-based methods produce corrosive waste. A green alternative to halides are naturally abundant carbonyl-based compounds called esters but these are inactive in palladium-catalyzed cross-coupling reactions that involve elimination of carbon monoxide.
To solve this issue, the team led by Magnus Rueping and Luigi Cavallo created a nickel catalyst that activates the cross-coupling of aromatic esters and alkyl boranes. In this reaction, the ester provides the aromatic portion of the product with or without a carbonyl group, while the borane is the alkyl precursor.
The researchers demonstrated that the reaction yielded a different family of products when they switched from one type of phosphorus ligand to another. Specifically, in the presence of a monodentate ligand, which is attached to the nickel center by a single bond, the reaction gave ketone derivatives. In contrast, ligands that formed two bonds with the metal promoted the production of alkyl arenes. “These experimental results were not easy to explain,” says Rueping. “Therefore, we used computational studies to help us understand the molecular mechanism and reaction pathway.”
This tunable approach was found to apply to a wide range of boranes and esters, such as benzene-like and heteroatom-containing aromatic derivatives. It generated essential intermediates in natural-product synthesis, such as potential next-generation antidepressants, and a potent antagonist for important cell adhesion proteins known as integrins.
“These results were exciting as most researchers would not have expected that this unusual site-selective reaction could be achieved by simply changing the ligands of the metal complex,” says Rueping. His team is currently studying other metal catalysts to simplify the synthesis of natural products and functional molecules.
Selected Publications
- Ligand-Controlled Chemoselective C(acyl)-O Bond vs C(aryl)-C Bond Activation of Aromatic Esters in Nickel Catalyzed...
A. Chatupheeraphat, H.-H. Liao, W. Srimontree, L. Guo, Y. Minenkov, A. Poater, L. Cavallo,...
J. Am. Chem. Soc., 140 (10), pp. 3724-3735, (2018)